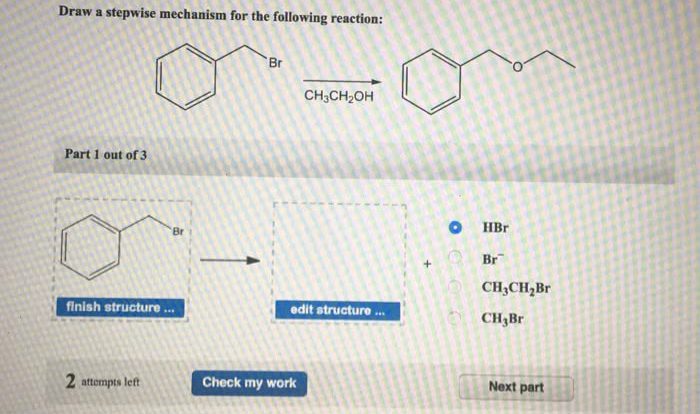
A rearrangement will occur in the carbocation intermediate shown. This rearrangement is a fundamental concept in organic chemistry that involves the structural rearrangement of carbocation intermediates during chemical reactions. Carbocations are highly reactive species that can undergo various rearrangements to achieve a more stable configuration.
The study of carbocation rearrangements is crucial for understanding the mechanisms of organic reactions and designing synthetic strategies. This article provides an overview of the concept, types, applications, and experimental methods used to investigate carbocation rearrangements.
Structural Rearrangements of Carbocation Intermediates: A Rearrangement Will Occur In The Carbocation Intermediate Shown
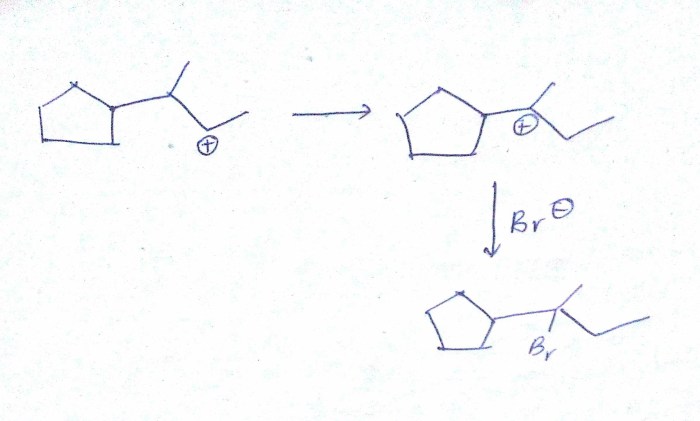
Carbocation intermediates are highly reactive species that can undergo a variety of rearrangements. These rearrangements are driven by the desire of the carbocation to achieve a more stable configuration. The most common types of carbocation rearrangements are 1,2-shifts, hydride shifts, and alkyl shifts.
1,2-shifts involve the movement of a group from one carbon atom to an adjacent carbon atom. Hydride shifts involve the movement of a hydrogen atom from one carbon atom to another. Alkyl shifts involve the movement of an alkyl group from one carbon atom to another.
The factors that influence the stability and reactivity of carbocations include the nature of the substituents and the reaction conditions. Carbocations that are substituted with electron-withdrawing groups are more stable than carbocations that are substituted with electron-donating groups. Carbocations that are formed in protic solvents are more stable than carbocations that are formed in aprotic solvents.
Types of Rearrangements
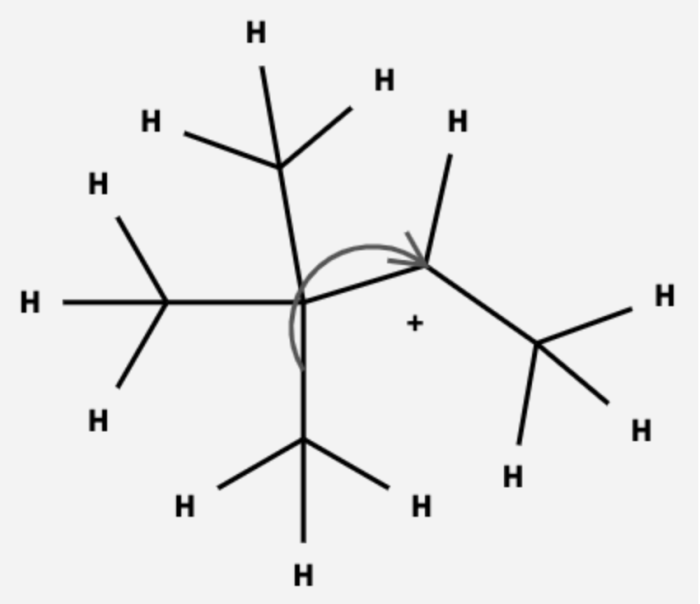
The different types of rearrangements that can occur in carbocation intermediates include:
- Wagner-Meerwein rearrangement:This rearrangement involves the migration of an alkyl group from one carbon atom to an adjacent carbon atom that is bonded to a heteroatom.
- Pinacol rearrangement:This rearrangement involves the migration of a hydroxyl group from one carbon atom to an adjacent carbon atom that is also bonded to a hydroxyl group.
- Demjanov rearrangement:This rearrangement involves the migration of a methyl group from one carbon atom to an adjacent carbon atom that is bonded to a tertiary carbon atom.
- Beckmann rearrangement:This rearrangement involves the migration of an aryl group from one carbon atom to an adjacent carbon atom that is bonded to a nitrogen atom.
Applications of Carbocation Rearrangements

Carbocation rearrangements are used in a variety of organic synthesis reactions. These reactions include:
- Regio- and stereoselective synthesis of complex molecules:Carbocation rearrangements can be used to regio- and stereoselectively synthesize complex molecules. This is because the regio- and stereochemistry of the carbocation intermediate can be controlled by the choice of starting materials and reaction conditions.
- Construction of cyclic compounds:Carbocation rearrangements can be used to construct cyclic compounds. This is because the carbocation intermediate can cyclize to form a new ring.
- Formation of functionalized alkenes and alkynes:Carbocation rearrangements can be used to form functionalized alkenes and alkynes. This is because the carbocation intermediate can react with nucleophiles to form new C-C bonds.
Experimental Methods for Studying Carbocation Rearrangements
The experimental techniques used to study carbocation rearrangements include:
- Kinetic studies:Kinetic studies can be used to determine the rate and mechanism of carbocation rearrangements. This is done by measuring the rate of disappearance of the starting material and the rate of appearance of the products.
- Isotopic labeling:Isotopic labeling can be used to track the movement of atoms during carbocation rearrangements. This is done by replacing one or more of the atoms in the starting material with an isotope of the same element.
- Computational modeling:Computational modeling can be used to predict the stability and reactivity of carbocations. This is done by using computer programs to calculate the energy of the carbocation intermediate and the transition states leading to the products.
Essential Questionnaire
What is a carbocation intermediate?
A carbocation intermediate is a positively charged carbon atom that forms during chemical reactions. It is a highly reactive species that can undergo rearrangements to achieve a more stable configuration.
What are the different types of carbocation rearrangements?
There are several types of carbocation rearrangements, including 1,2-shifts, hydride shifts, alkyl shifts, Wagner-Meerwein rearrangements, Pinacol rearrangements, Demjanov rearrangements, and Beckmann rearrangements.
What factors influence the stability of carbocations?
The stability of carbocations is influenced by several factors, including the nature of the substituents, the resonance effects, and the hybridization of the carbon atom.